Life requires carbon.
Every day, you need to eat about 250g of carbon to live.[1]
That may not sound like much, but to feed every human on the planet, plants have to provide 600 million metric tons of carbon each year! (And that’s if we were all vegetarians)
But don’t worry! The atmosphere contains over 2 billion metric tons of carbon right now, and we’re increasing that number every day![2]
There’s just one more catch: atmospheric carbon is CO2 – the fully oxidized form of carbon, which is totally useless to us.
Fortunately, there is a protein that can help!
RuBisCO!
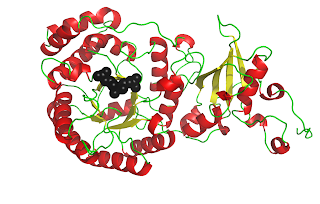
RuBisCO[3] catalyzes the carbon-fixing reaction in which a molecule of CO2 is added to the enediol form of ribulose-1,5-bisphosphate (RuBP), forming two molecules of 3-phospoglyceraldehyde (3-PGA). In these figures, you can see a crystal structure of the unactivated form of RuBisCO bound to its substrate RuBP[4] (above) and the activated form of RuBisCO bound to its two 3-PGA products[5] (below). Notice how the protein chain has undergone a conformational change in the activated complex, bringing the beta sheet behind the beta barrel. Also, activated RuBisCO contains a magnesium ion, shown in blue. These images show just one of the sixteen subunits of RuBisCO. For a better understanding of RuBisCO’s structure, see the “RuBisCO Images” post[6].
Let me give you four words to help you remember RuBisCO’s characteristics:
Pervasive: RuBisCO is the most abundant protein on the planet.
Perseverant: Day in and day out it, it works diligently to provide you with the carbon you need to live. Although RuBisCO can perform only three reactions a second, it keeps right on working, fixing carbon to give you delicious sweet corn, crisp cucumbers and juicy watermelon all summer long.
Practically Perfect: Some have accused RuBisCO of substrate promiscuity, arguing that a more ideal protein would be able to better discriminate between CO2 and O2, but all efforts to improve this protein have failed! In fact, RuBisCO accomplishes a reaction that no chemist has ever accomplished: efficiently fixing CO2 straight from the atmosphere! This means it has to discriminate between CO2 and O2, two nearly featureless gases. Additionally, the transition state for fixing CO2 is higher energy than the transition state for fixing O2, which means that increasing the enzyme’s specificity for CO2 means decreasing its rate. Each RuBisCO is highly tuned to the CO2:O2 ratios and temperatures in its plant’s environment so that it balances specificity and speed. RuBisCO has not been improved because it is practically perfect!
Pervasive. Perseverant. Practically Perfect.
Show your appreciation to the diligent protein that fixes life.
Vote RuBisCO
[1] My calculations based on recommended daily values for an average college student from http://www.healthcalculators.org/index.html (313g carbohydrates, 85g protein, 69g fat) and percent carbon composition of carbohydrates (40%), proteins (~50%) and fats (75%).
[3] Information about RuBisCO was attained from the papers sited at http://rubiscofixeslife.blogspot.com/2011/03/all-about-rubisco.html.
[4] PDB file 1RCX
[5] PDB file 1AA1